Tramadol hydrochloride is a treatment option for the management of pain1
Lowest approved strength to initiate the pain management journey
Titration with Tramadol 25 mg offers a lower incidence of discontinuation due to adverse events
Improved tolerability with a lower adverse event profile
Tapering with lowest effective strength to mitigate withdrawal symptoms
It is highly encouraged that healthcare providers complete a REMS-compliant education program and counsel their patients and caregivers regarding serious risks, safe use, and the significance of medication guide.
About Tramadol
Efficient Titration - No more guesswork
With the newly introduced doses of 25 mg and 75 mg, clinicians may titrate dose of tramadol efficiently.
- Tramadol 25 mg is the lowest approved and available strength that gives clinicians an option of low-dose titration to suit individual patient needs. This will avoid current practice of splitting tablets of higher strengths into smaller portions, giving better dose accuracy.
- With tramadol 75 mg tablets, clinicians may titrate and taper in lowest possible dose options. The dose can be easily titrated up from 50 mg to 75 mg or tapered down from 100 mg to 75 mg, so the patients receive the lowest effective dosage at any given time of treatment regimen.

INDICATIONS AND USAGE
Tramadol hydrochloride is an opioid agonist indicated in adults for the management of pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate.1
Limitations of Use:
Because of the risks of addiction, abuse, and misuse with opioids, which can occur at any dosage or duration, reserve tramadol hydrochloride tablets for use in patients for whom alternative treatment options (e.g., non-opioid analgesics or opioid combination products):
- Have not been tolerated or are not expected to be tolerated.
- Have not provided adequate analgesia or are not expected to provide adequate analgesia.
Tramadol hydrochloride tablets should not be used for an extended period unless the pain remains severe enough to require an opioid analgesic and for which alternative treatment options continue to be inadequate.1
Click here for full Prescribing Information including Boxed WARNING.
Mechanism of Action
Tramadol hydrochloride tablets contain tramadol, an opioid agonist and inhibitor of norepinephrine and serotonin reuptake. The analgesic effect of tramadol is believed to be due to both binding to μ-opioid receptors and weak inhibition of reuptake of norepinephrine and serotonin (Figure 1).1
Opioid activity is due to both low-affinity binding of the parent compound and high-affinity binding of the O-demethylated metabolite M1 to μ-opioid receptors. Analgesia in humans begins approximately within 1 hour after administration and reaches a peak in approximately 2-3 hours.1
The figure shows how tramadol works by binding to μ-opioid receptors in the brain and inhibiting the reuptake of norepinephrine and serotonin, which helps to alleviate pain.
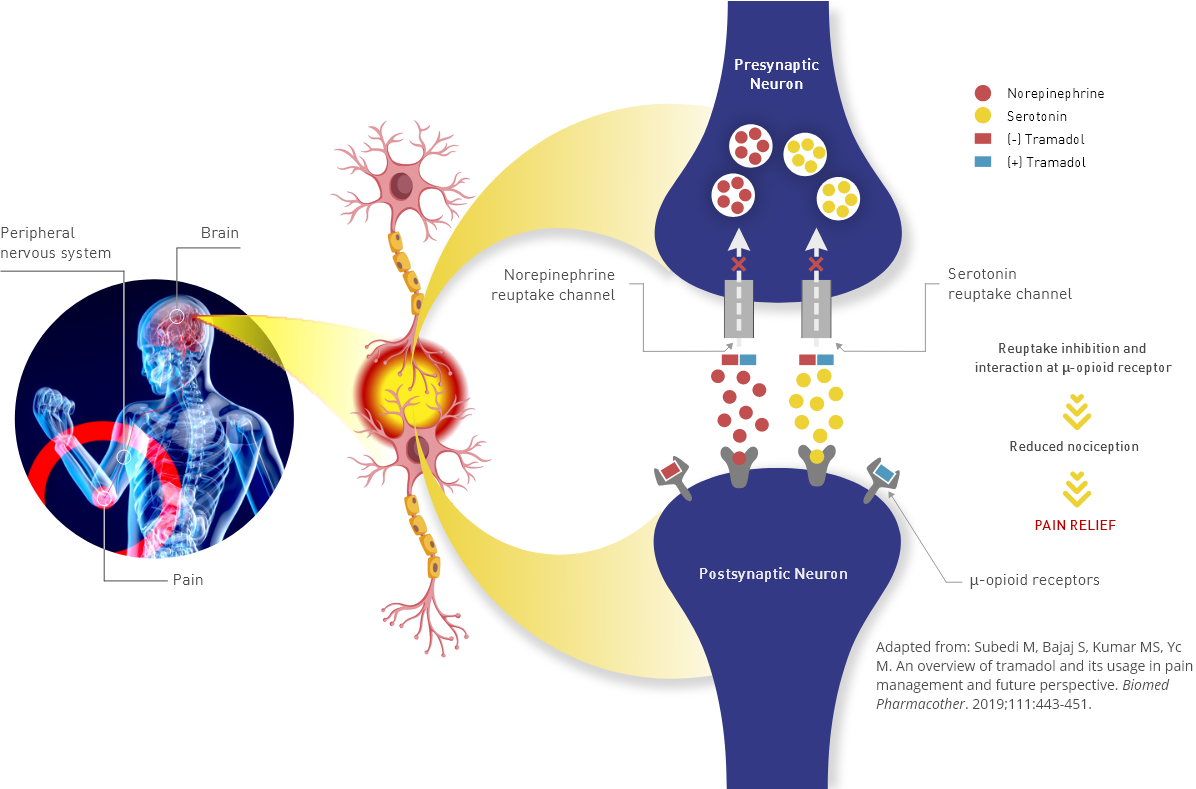
Figure 1. Schematic Representation
of the Mechanism of Action of Tramadol Hydrochloride2
Figure 1. Schematic Representation of the Mechanism of Action of Tramadol Hydrochloride2
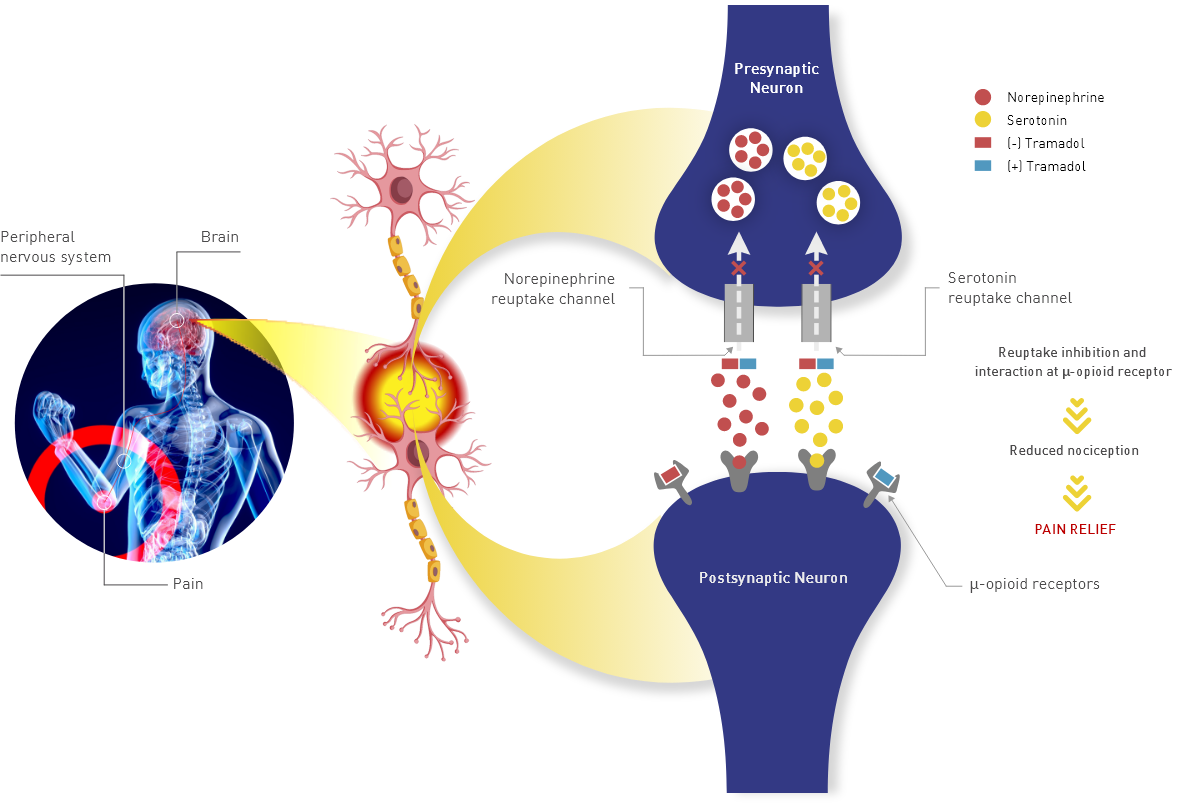
Adapted from: Subedi M, Bajaj S, Kumar MS, Yc M. An overview of tramadol and its usage in pain management and future perspective. Biomed Pharmacother. 2019;111:443-451.
Dosage and Administration
Tramadol hydrochloride tablets, USP for oral use, are supplied as tablets for oral administration in 25, 50, 75 and 100 mg strengths.1
Tramadol 25 mg: Titrate to an optimal therapeutic dose1
Consider Tramadol 25 mg to plan tailored treatment regimen using a slow titration approach and 75 mg tablets to individualize dose.
INITIATE
Initiate treatment at the lowest dose necessary to achieve adequate analgesia. Titrate the dose based upon the individual patient’s response to their initial dose of tramadol hydrochloride tablets. Consider starting tramadol hydrochloride tablets at 25 mg/day, in patients not requiring rapid onset of analgesia.
TITRATE
Consider titrating in 25 mg increments as separate doses every 3 days to reach 100 mg/day (25 mg four times a day). Thereafter the total daily dose may be increased by 50 mg as tolerated every 3 days to reach 200 mg/day (50 mg four times a day).
MAINTAIN
After titration, tramadol hydrochloride tablets 50 mg to 100 mg can be administered as needed for pain relief every 4 to 6 hours, not exceeding 400 mg/day.
For patients who need rapid onset of analgesia and for whom the benefits outweigh the risk of discontinuation due to adverse effect, consider tramadol hydrochloride tablets 50 mg to 100 mg as needed for pain relief every 4 to 6 hours, not exceeding 400 mg per day.
TAPER
Consider slow tapering to achieve safe reduction or discontinuation in patients who may be physically dependent on opioids to avoid serious withdrawal symptoms, uncontrolled pain, and suicide.
Key points to consider before prescribing tramadol hydrochloride


- Use the lowest effective dosage for the shortest duration consistent with each patient’s treatment goals.1
- Initiate the dosing regimen for each patient individually, considering the patient’s severity of pain, patient response, prior analgesic treatment experience, and risk factors for addiction, abuse, and misuse.1
- Monitor patients closely for respiratory depression, especially within the first 24-72 hours of initiating therapy and following dosage increases with tramadol hydrochloride tablets, and adjust the dosage accordingly.1
Dose individualization
According to the CDC’s Clinical Practice Guideline for Prescribing Opioids for Pain,
- When the diagnosis and severity of acute pain warrant use of opioids, clinicians should prescribe no greater quantity than needed for the expected duration of pain severe enough to require opioids.3
- For many common causes of nontraumatic, nonsurgical pain, when opioids are needed, a few days or less are often sufficient, and shorter courses can minimize the need to taper opioids to prevent withdrawal symptoms at the end of a course of opioids.3
- In the light of health inequities reported, clinicians should reduce disparities in pain management care across age, ethnicity, socioeconomic groups, and geographies using multimodal and multidisciplinary approaches such as tailored interventions and strategies addressing chronic pain in the United States.3
Efficacy
Tramadol hydrochloride is proven therapy for pain management. Efficacy of tramadol hydrochloride has been clinically studied and is a proven treatment option for pain severe enough to require opioid analgesic and for which alternative treatments are inadequate.1


Safety and Tolerability
Tramadol 25 mg reduced the probability of adverse reactions leading to less occurrence of treatment discontinuations1,4
Tramadol hydrochloride was studied in 3 dosage groups employed in 10-, 16-, or a 13-day titration schedule in patients who were previously intolerant to tramadol hydrochloride. The 3 treatment groups (protocol regimens) are as shown in Table 1.4
Table 1. Study Medication Dosage and Titration Schedules During the Double-Blind Phase4
| Treatment group (protocol regimen #) | Dose of tramadol hydrochloride by study days | |||||
|---|---|---|---|---|---|---|
| 1-3 | 4-6 | 7-9 | 10-12 | 13-15 | 16-28 | |
| 10 days to 200 mg | 50 mg (AM) | 50 mg b.i.d. | 50 mg t.i.d. | 50 mg q.i.d. | 50 mg q.i.d. | 50 mg q.i.d. |
| 16 days to 200 mg | 25 mg (AM) | 25 mg b.i.d. | 25 mg t.i.d. | 25 mg q.i.d. | 50 mg t.i.d. | 50 mg q.i.d. |
| 13 days to 150 mg | 25 mg (AM) | 25 mg b.i.d. | 25 mg t.i.d. | 25 mg q.i.d. | 50 mg t.i.d. | 50 mg t.i.d. |
Adapted from: Petrone D, Kamin M, Olson W. Slowing the titration rate of tramadol HCl reduces the incidence of discontinuation due to nausea and/or vomiting: a double-blind randomized trial. J Clin Pharm Ther. 1999;24(2):115-123.
Slow titration was associated with fewer adverse reactions and discontinuations
There were fewer adverse events reported in 13-day titration schedule when compared with 10-day and 16-day titration schedules (33 vs 41 and 41).4
There were significantly fewer discontinuations due to nausea and/or vomiting when the titration rate was slow, and when the titration was initiated with tramadol hydrochloride 25 mg and was gradually increased by 25 mg every 3 days.4
The 13- and 16-day tramadol hydrochloride titration groups compared to the 10-day group showed a significantly lower percentage of discontinuations due to nausea and/or vomiting (22%; P = 0.006 and P = 0.008) (Figure 2(A) and 2(B)). Similar results were observed in terms of the percentage of patients discontinuing due to any adverse event.4
Figure 2(A). A Summary of Double-Blind Discontinuations in Each Treatment Group Due to Nausea and/or Vomiting4
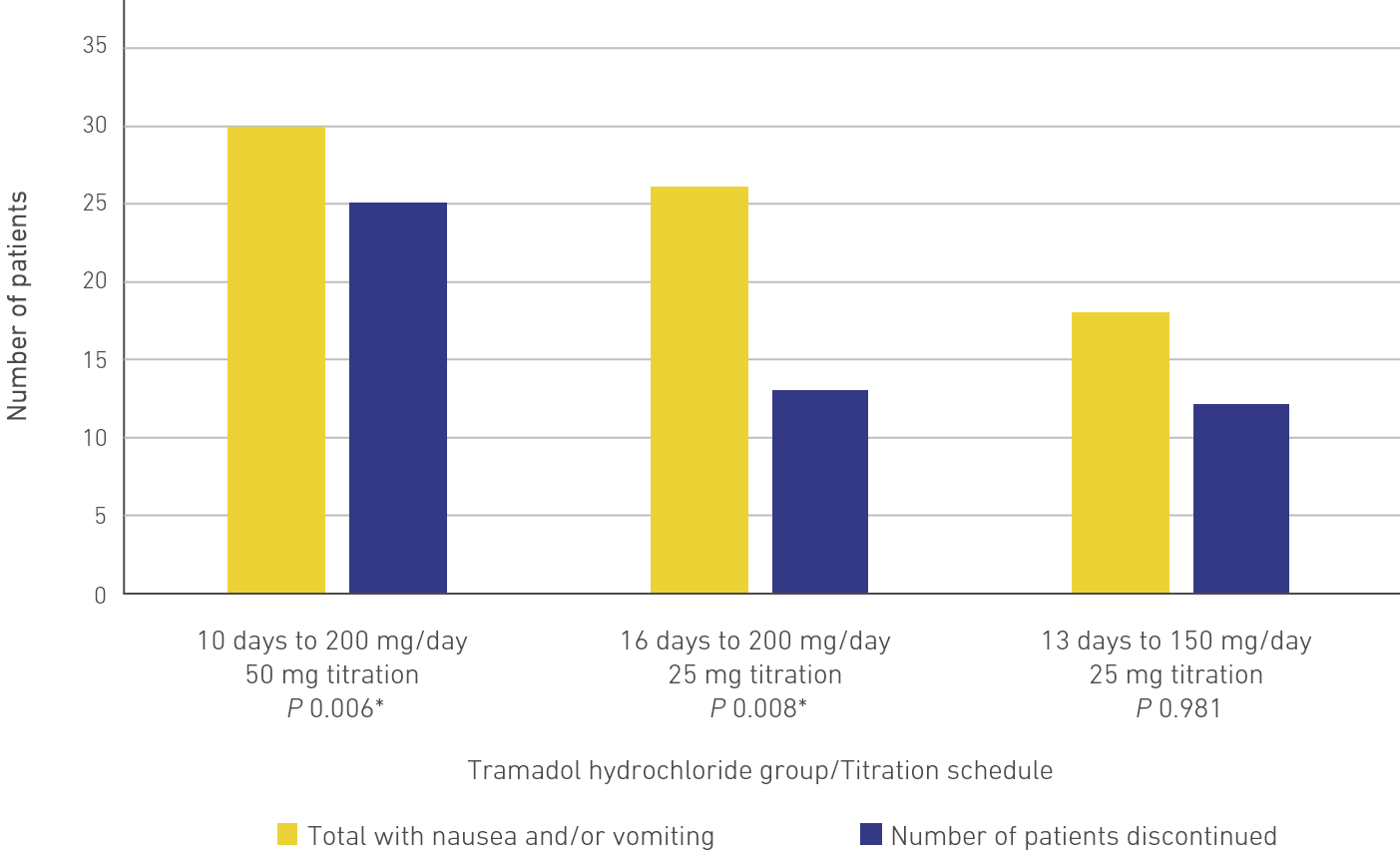
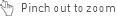
Figure 2(B). A Summary of Double-Blind Discontinuations in Each Treatment Group Due to Any Adverse Event4
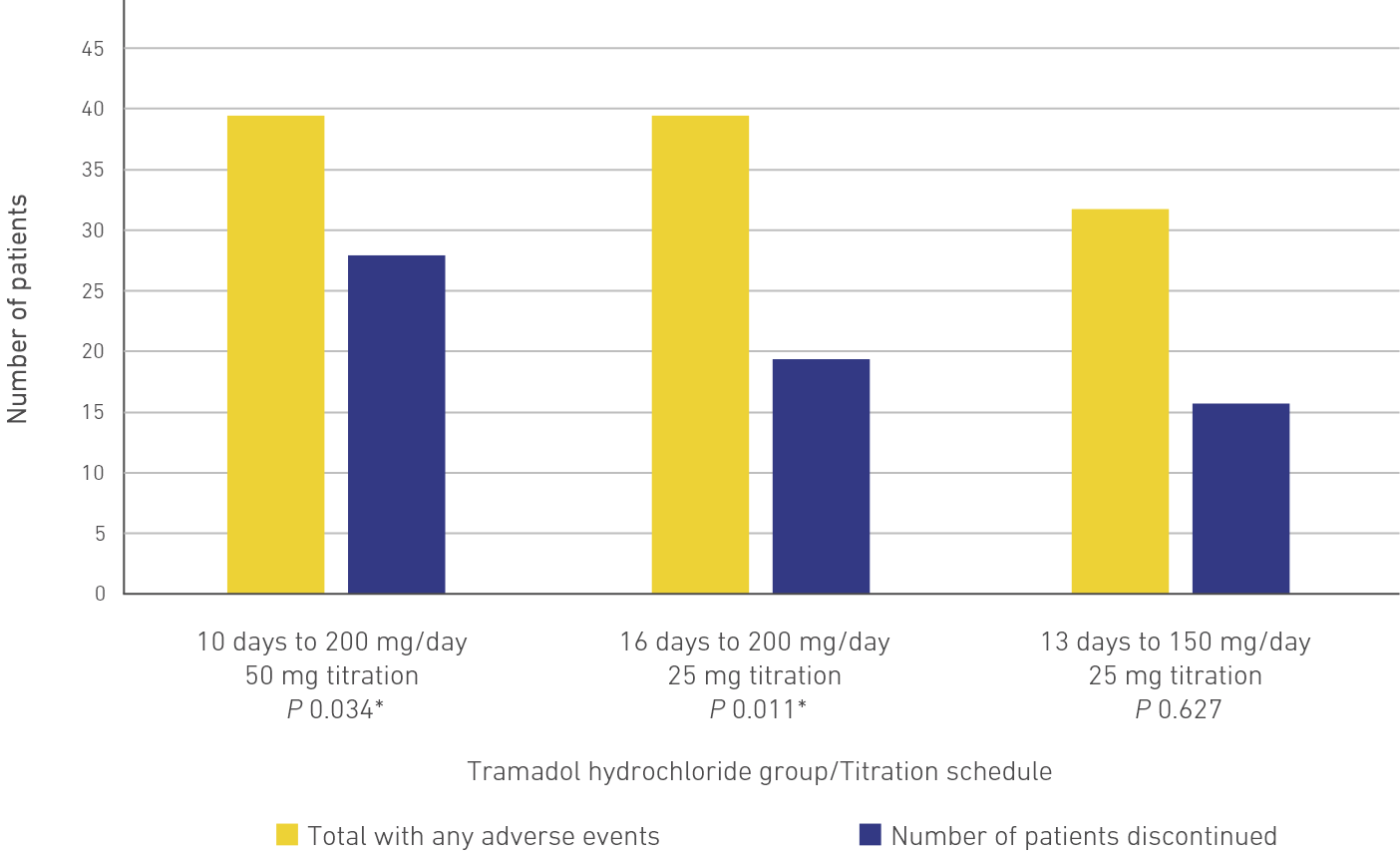
*Significant at P < 0.05.
Adapted from: Petrone D, Kamin M, Olson W. Slowing the titration rate of tramadol HCl reduces the incidence of discontinuation due to nausea and/or vomiting: a double-blind randomized trial. J Clin Pharm Ther. 1999;24(2):115-123.
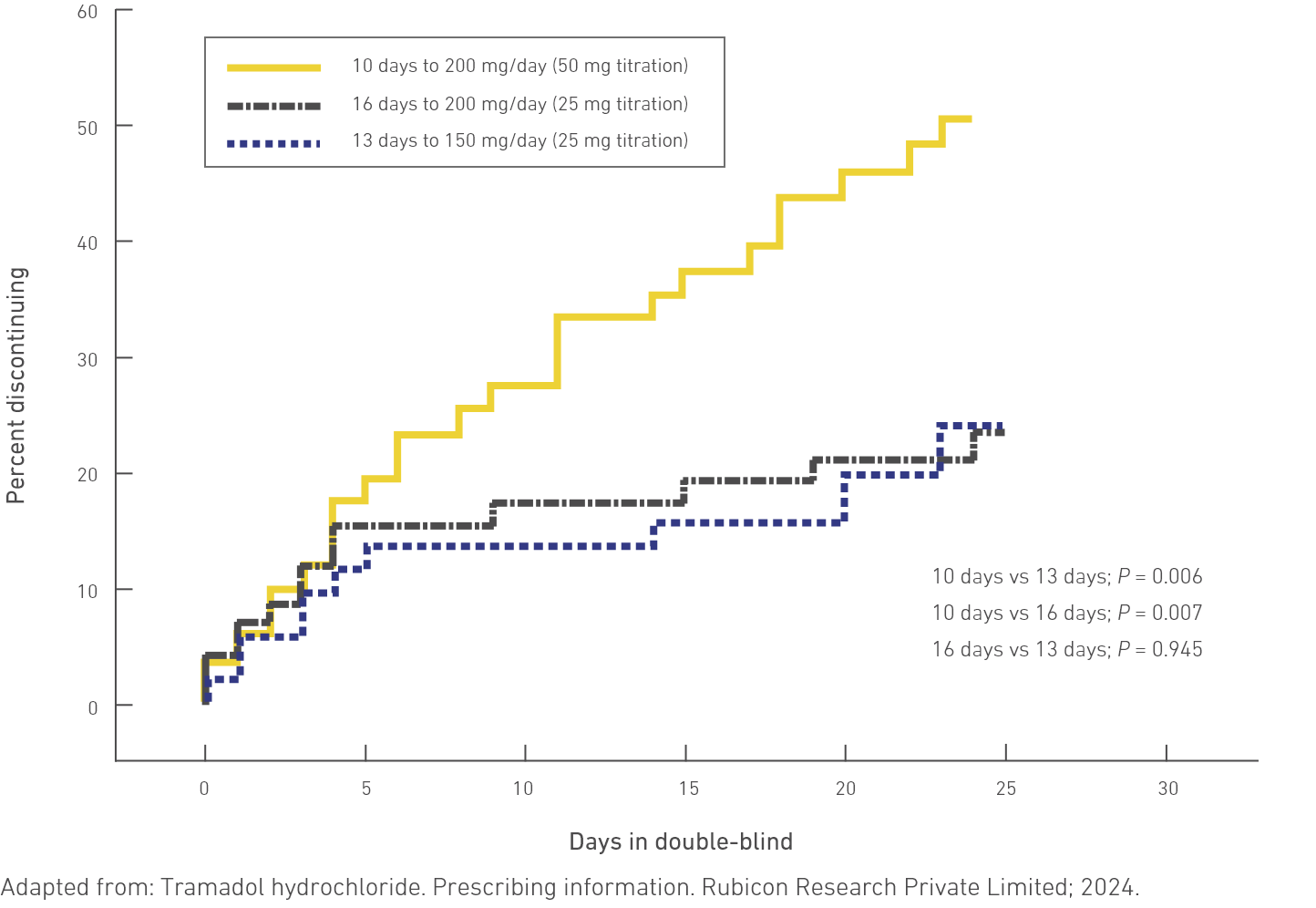
The Kaplan–Meier survival curves show reduced probability of discontinuation due to nausea and/or vomiting4
Time to discontinuation for nausea and/or vomiting was significantly longer in the 13- and 16-day groups compared with the 10-day group (P = 0.006 and P = 0.007), respectively (Figure 3).
The Kaplan–Meier survival curves show that the cumulative probability of discontinuation due to nausea and/or vomiting in the 3 titration groups is similar through the first 5 days of titration.
After day 5, the cumulative probability of discontinuation due to nausea and/or vomiting in the 10-day titration group continued to increase at a rate similar to that seen in the first 5 days, whereas the 16- and 13-day titration groups plateaued.4
Figure 3. The Kaplan–Meier Survival Curves of the Time to Discontinuation Due to Nausea and/or Vomiting
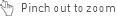
Tramadol 25 mg has an improved safety and tolerability profile1,2
Tramadol hydrochloride exhibits minimal serious adverse effects without the potential for dependency when used in therapeutic doses under medical advice, unlike other opioids.2
Tramadol hydrochloride was studied in 550 patients during the double-blind or open-label extension periods in studies of chronic nonmalignant pain, in the United States. Of these patients, 375 were 65 years old or older. Tramadol hydrochloride was compared with 2 active control groups: acetaminophen 300 mg with codeine phosphate 30 mg, and aspirin 325 mg with codeine phosphate 30 mg.1
Table 2 reports the cumulative incidence rate of adverse reactions by 7, 30, and 90 days for the most common adverse reactions (occurring in 5% or more of cases by 7 days).1
- The most frequently reported adverse events were related to the central nervous system and gastrointestinal system.1
- The overall incidence rates of adverse events in these trials were similar between the group receiving tramadol hydrochloride tablets and the active control groups.1
Table 2. The Cumulative Incidence of Adverse Reactions for Tramadol Hydrochloride Tablets in Trials of Patients With Chronic Nonmalignant Pain (N = 427)1
| Up to 7 days | Up to 30 days | Up to 90 days | |
|---|---|---|---|
| Dizziness/Vertigo | 26% | 31% | 33% |
| Nausea | 24% | 34% | 40% |
| Constipation | 24% | 38% | 46% |
| Headache | 18% | 26% | 32% |
| Somnolence | 16% | 23% | 25% |
| Vomiting | 9% | 13% | 17% |
| Pruritus | 8% | 10% | 11% |
| CNS stimulation* | 7% | 11% | 14% |
| Asthenia | 6% | 11% | 12% |
| Sweating | 6% | 7% | 9% |
| Dyspepsia | 5% | 9% | 13% |
| Dry mouth | 5% | 9% | 10% |
| Diarrhea | 5% | 6% | 10% |
CNS, central nervous system.
CNS Stimulation is a composite of nervousness, anxiety, agitation, tremor, spasticity, euphoria, emotional lability and hallucinations.
Adapted from: Tramadol hydrochloride. Prescribing information. Rubicon Research Private Limited; 2024.
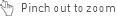
Key warnings for tramadol hydrocholoride1
WARNINGS
- Opioid-Induced Hyperalgesia and Allodynia: Opioid-Induced Hyperalgesia (OIH) occurs when an opioid analgesic paradoxically causes an increase in pain, or an increase in sensitivity to pain. If a patient is suspected to be experiencing OIH, carefully consider appropriately decreasing the dose of the current opioid analgesic, or opioid rotation (safely switching the patient to a different opioid moiety).
- Serotonin Syndrome: May be life-threatening. Can occur with the use of tramadol alone, with the concomitant use of serotonergic drugs, or with drugs that impair metabolism of serotonin or tramadol.
- Risk of Seizure: Can occur at the recommended dose of tramadol. Concomitant use with other drugs may increase the risk of seizures. The risk may increase in patients with epilepsy, a history of seizures, and in patients with a recognized risk for seizures.
- Risk of Suicide: Do not prescribe for suicidal or addiction-prone patients.
- Adrenal Insufficiency: If diagnosed, treat with physiologic replacement of corticosteroids and gradually reduce the patient's opioid usage.
- Life-threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients: Regularly evaluate closely, particularly during dosage initiation and titration.
- Severe Hypotension: Regularly evaluate during dosage initiation and titration. Avoid use of tramadol hydrochloride tablets in patients with circulatory shock.
- Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness: Regularly evaluate for sedation and respiratory depression. Avoid use of tramadol hydrochloride tablets in patients who are experiencing impaired consciousness or are in a coma.
Click here for full Prescribing Information including Boxed WARNING.
References:
- TRAMADOL HYDROCHLORIDE. Prescribing information. Rubicon Research Private Limited; 2024.
- Subedi M, Bajaj S, Kumar MS, Yc M. An overview of tramadol and its usage in pain management and future perspective. Biomed Pharmacother. 2019;111:443-451.
- Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC Clinical Practice Guideline for Prescribing Opioids for Pain - United States, 2022. MMWR Recomm Rep. 2022;71(3):1-95.
- Petrone D, Kamin M, Olson W. Slowing the titration rate of tramadol HCl reduces the incidence of discontinuation due to nausea and/or vomiting: a double-blind randomized trial. J Clin Pharm Ther. 1999;24(2):115-123.
INDICATION AND IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS AND LIFE-THREATENING RISKS FROM USE OF TRAMADOL HYDROCHLORIDE TABLETS
See full prescribing information for complete boxed warning
- Tramadol hydrochloride tablets exposes users to the risks of addiction, abuse and misuse, which can lead to overdose and death. Assess each patient’s risk prior to prescribing tramadol hydrochloride tablets, and monitor regularly for these behaviors or conditions.
- To ensure that the benefits of opioid analgesics outweigh the risks of addiction, abuse, and misuse, the Food and Drug Administration (FDA) has required a Risk Evaluation and Mitigation Strategy (REMS) for these products.
- Serious, life-threatening, or fatal respiratory depression may occur. Monitor closely, especially during initiation or following a dose increase.
- Accidental ingestion of tramadol hydrochloride tablets, especially by children, can result in a fatal overdose of tramadol.
- Life-threatening respiratory depression and death have occurred in children who received tramadol. Some of the reported cases followed tonsillectomy and/or adenoidectomy; in at least one case, the child had evidence of being an ultra-rapid metabolizer of tramadol due to a CYP2D6 polymorphism.
- Tramadol hydrochloride tablets are contraindicated in children younger than 12 years of age and in children younger than 18 years of age following tonsillectomy and/or adenoidectomy. Avoid the use of tramadol hydrochloride tablets in adolescents 12 to 18 years of age who have other risk factors that may increase their sensitivity to the respiratory depressant effects of tramadol.
- Prolonged use of tramadol hydrochloride tablets, during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life threatening if not recognized and treated if prolonged opioid use is required in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available.
- The effects of concomitant use or discontinuation of cytochrome P450 3A4 inducers, 3A4 inhibitors, or 2D6 inhibitors with tramadol are complex. Use of cytochrome P450 3A4 inducers, 3A4 inhibitors, or 2D6 inhibitors with tramadol hydrochloride tablets require careful consideration of the effects on the parent drug, tramadol, and the active metabolite, M1.
- Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing for use in patients for whom alternative treatment options are inadequate; limit dosages and durations to the minimum required; and follow patients for signs and symptoms of respiratory depression and sedation
Indications and Usage
Tramadol hydrochloride is an opioid agonist indicated in adults for the management of pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate.
Limitations of Use:
Because of the risks of addiction, abuse, and misuse with opioids, which can occur at any dosage or duration, reserve tramadol hydrochloride tablets for use in patients for whom alternative treatment options (e.g., non-opioid analgesics or opioid combination products):
- Have not been tolerated or are not expected to be tolerated.
- Have not provided adequate analgesia or are not expected to provide adequate analgesia.
Tramadol hydrochloride tablets should not be used for an extended period of time unless the pain remains severe enough to require an opioid analgesic and for which alternative treatment options continue to be inadequate.
CONTRAINDICATIONS
- Children younger than 12 years of age.
- Post-operative management in children younger than 18 years of age following tonsillectomy and/or adenoidectomy.
- Significant respiratory depression.
- Acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment.
- Known or suspected gastrointestinal obstruction, including paralytic ileus.
- Hypersensitivity to tramadol, any other component of this product or opioids.
- Concurrent use of monoamine oxidase inhibitors (MAOIs) or use of MAOIs within the last 14 days.
WARNINGS AND PRECAUTIONS
- Opioid-Induced Hyperalgesia and Allodynia: Opioid-Induced Hyperalgesia (OIH) occurs when an opioid analgesic paradoxically causes an increase in pain, or an increase in sensitivity to pain. If a patient is suspected to be experiencing OIH, carefully consider appropriately decreasing the dose of the current opioid analgesic, or opioid rotation (safety switching the patient to a different opioid moiety).
- Serotonin Syndrome: May be life-threatening. Can occur with use of tramadol alone, with concomitant use of serotonergic drugs, with drugs that impair metabolism of serotonin or tramadol.
- Risk of Seizure: Can occur at the recommended dose of tramadol. Concomitant use with other drugs may increase seizure risk. Risk may increase in patients with epilepsy, a history of seizures, and in patients with a recognized risk for seizures.
- Risk of Suicide: Do not prescribe for suicidal or addiction-prone patients.
- Adrenal Insufficiency: If diagnosed, treat with physiologic replacement of corticosteroids, and wean patient off the opioid.
- Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients: Regularly evaluate closely, particularly during initiation and titration.
- Severe Hypotension: Regularly evaluate during dosage initiation and titration. Avoid use of tramadol hydrochloride tablets in patients with circulatory shock.
- Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness: Regularly evaluate for sedation and respiratory depression. Avoid use of tramadol hydrochloride tablets in patients with impaired consciousness or coma.
ADVERSE REACTIONS
The most common incidence of treatment-emergent adverse events (≥15.0%) in patients from clinical trials were dizziness/vertigo, nausea, constipation, headache, somnolence, vomiting and pruritus.
To report SUSPECTED ADVERSE REACTIONS, contact AdvaGen Pharma Ltd, at 1-866-488-0312 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Mixed Agonist/Antagonist and Partial Agonist Opioid Analgesics: Avoid use with tramadol hydrochloride tablets because they may reduce analgesic effect of tramadol hydrochloride tablets or precipitate withdrawal symptoms.
USE IN SPECIFIC POPULATIONS
- Pregnancy: May cause fetal harm.
- Lactation: Breastfeeding not recommended.
Please click here for full Prescribing Information including Boxed WARNING and Medication Guide.
INDICATION AND IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS AND LIFE-THREATENING RISKS FROM USE OF TRAMADOL HYDROCHLORIDE TABLETS
See full prescribing information for complete boxed warning
- Tramadol hydrochloride tablets exposes users to the risks of addiction, abuse and misuse, which can lead to overdose and death. Assess each patient’s risk prior to prescribing tramadol hydrochloride tablets, and monitor regularly for these behaviors or conditions.
- To ensure that the benefits of opioid analgesics outweigh the risks of addiction, abuse, and misuse, the Food and Drug Administration (FDA) has required a Risk Evaluation and Mitigation Strategy (REMS) for these products.
- Serious, life-threatening, or fatal respiratory depression may occur. Monitor closely, especially during initiation or following a dose increase.
- Accidental ingestion of tramadol hydrochloride tablets, especially by children, can result in a fatal overdose of tramadol.
- Life-threatening respiratory depression and death have occurred in children who received tramadol. Some of the reported cases followed tonsillectomy and/or adenoidectomy; in at least one case, the child had evidence of being an ultra-rapid metabolizer of tramadol due to a CYP2D6 polymorphism.
- Tramadol hydrochloride tablets are contraindicated in children younger than 12 years of age and in children younger than 18 years of age following tonsillectomy and/or adenoidectomy. Avoid the use of tramadol hydrochloride tablets in adolescents 12 to 18 years of age who have other risk factors that may increase their sensitivity to the respiratory depressant effects of tramadol.
- Prolonged use of tramadol hydrochloride tablets, during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life threatening if not recognized and treated if prolonged opioid use is required in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available.
- The effects of concomitant use or discontinuation of cytochrome P450 3A4 inducers, 3A4 inhibitors, or 2D6 inhibitors with tramadol are complex. Use of cytochrome P450 3A4 inducers, 3A4 inhibitors, or 2D6 inhibitors with tramadol hydrochloride tablets require careful consideration of the effects on the parent drug, tramadol, and the active metabolite, M1.
- Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing for use in patients for whom alternative treatment options are inadequate; limit dosages and durations to the minimum required; and follow patients for signs and symptoms of respiratory depression and sedation.
Indications and Usage
Tramadol hydrochloride is an opioid agonist indicated in adults for the management of pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate.
Limitations of Use:
Because of the risks of addiction, abuse, and misuse with opioids, which can occur at any dosage or duration, reserve tramadol hydrochloride tablets for use in patients for whom alternative treatment options (e.g., non-opioid analgesics or opioid combination products):
- Have not been tolerated or are not expected to be tolerated.
- Have not provided adequate analgesia or are not expected to provide adequate analgesia.
Tramadol hydrochloride tablets should not be used for an extended period of time unless the pain remains severe enough to require an opioid analgesic and for which alternative treatment options continue to be inadequate.
CONTRAINDICATIONS
- Children younger than 12 years of age.
- Post-operative management in children younger than 18 years of age following tonsillectomy and/or adenoidectomy.
- Significant respiratory depression.
- Acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment.
- Known or suspected gastrointestinal obstruction, including paralytic ileus.
- Hypersensitivity to tramadol, any other component of this product or opioids.
- Concurrent use of monoamine oxidase inhibitors (MAOIs) or use of MAOIs within the last 14 days.
WARNINGS AND PRECAUTIONS
- Opioid-Induced Hyperalgesia and Allodynia: Opioid-Induced Hyperalgesia (OIH) occurs when an opioid analgesic paradoxically causes an increase in pain, or an increase in sensitivity to pain. If a patient is suspected to be experiencing OIH, carefully consider appropriately decreasing the dose of the current opioid analgesic, or opioid rotation (safety switching the patient to a different opioid moiety).
- Serotonin Syndrome: May be life-threatening. Can occur with use of tramadol alone, with concomitant use of serotonergic drugs, with drugs that impair metabolism of serotonin or tramadol.
- Risk of Seizure: Can occur at the recommended dose of tramadol. Concomitant use with other drugs may increase seizure risk. Risk may increase in patients with epilepsy, a history of seizures, and in patients with a recognized risk for seizures.
- Risk of Suicide: Do not prescribe for suicidal or addiction-prone patients.
- Adrenal Insufficiency: If diagnosed, treat with physiologic replacement of corticosteroids, and wean patient off the opioid.
- Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients: Regularly evaluate closely, particularly during initiation and titration.
- Severe Hypotension: Regularly evaluate during dosage initiation and titration. Avoid use of tramadol hydrochloride tablets in patients with circulatory shock.
- Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness: Regularly evaluate for sedation and respiratory depression. Avoid use of tramadol hydrochloride tablets in patients with impaired consciousness or coma.
ADVERSE REACTIONS
The most common incidence of treatment-emergent adverse events (≥15.0%) in patients from clinical trials were dizziness/vertigo, nausea, constipation, headache, somnolence, vomiting and pruritus.
To report SUSPECTED ADVERSE REACTIONS, contact AdvaGen Pharma Ltd, at 1-866-488-0312 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Mixed Agonist/Antagonist and Partial Agonist Opioid Analgesics: Avoid use with tramadol hydrochloride tablets because they may reduce analgesic effect of tramadol hydrochloride tablets or precipitate withdrawal symptoms.
USE IN SPECIFIC POPULATIONS
- Pregnancy: May cause fetal harm.
- Lactation: Breastfeeding not recommended.
Please click here for full Prescribing Information including Boxed WARNING and Medication Guide.

50 Millstone Road, Building 200, Suite 180 East Windsor, New Jersey, 08520,
Contact AdvaGen Pharma Ltd. at tramadol25@advagenpharma.com
Visit us at advagenpharma.com
Toll-free number: 1-888-889-3009


